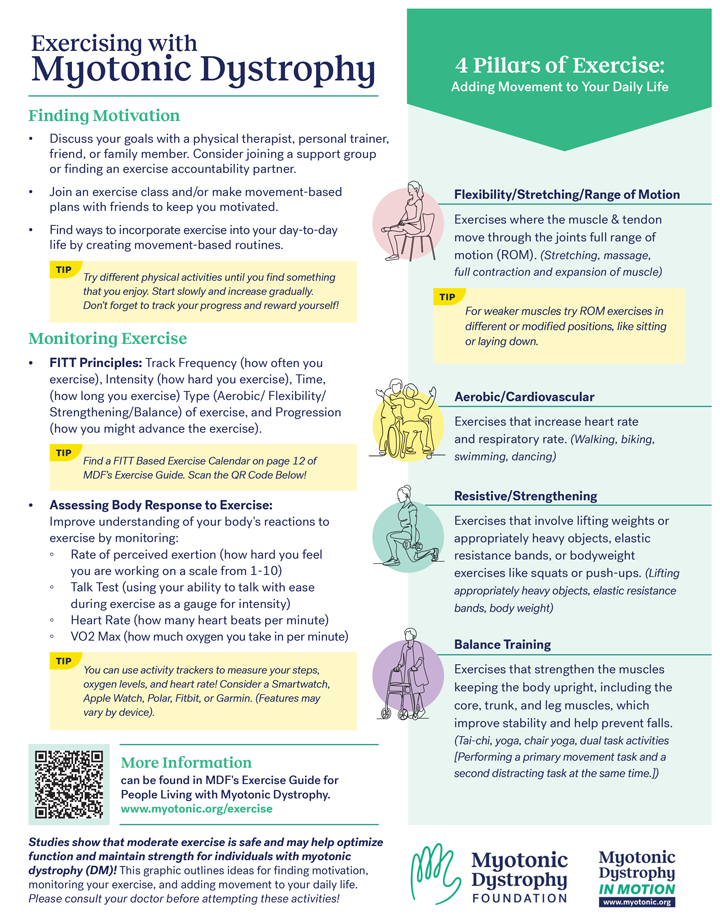
Bringing Myotonic Dystrophy into the Newborn Screening Conversation
On February 14, 2025, the RNA Institute and the Myotonic Dystrophy Foundation (MDF) organized and co-hosted the first ever symposium exploring how to include myotonic dystrophy (DM) in newborn screening. Newborn screening is one of the most successful public health services testing all babies shortly after birth for certain genetic, life-threatening diseases. Conditions included in newborn screening are serious and life threatening and without early diagnosis would result in irreparable harm and death. Conditions included in newborn screening are also diseases that do not have treatments but benefit from early disease management thereby significantly improving clinical outcomes. Newborn screening identifies babies at risk for these diseases before symptoms start, ensures the best possible outcomes for all babies, and limits pain and suffering.
Click here to download the agenda from the Newborn Screening & Sequencing for Myotonic Dystrophy Mini-Symposium 2025! >>>
Looking for more detail? Scroll down for scientific and expert-level summaries, including a Scientific Abstract and a deeper technical overview.
The Power and Promise of Genome Sequencing
The meeting brought together researchers, doctors, public health leaders, industry experts, and patient advocates to share progress in genome sequencing, diagnostic tools, and pilot newborn screening programs. Laboratory tests using genome sequencing allow the identification of a select set of genetic diseases based on changes in a person’s DNA. Diagnostic genome sequencing is a comprehensive genetic test performed in a specialized laboratory. The test analyzes an individual's entire genome or DNA to identify potential disease-causing changes. It is increasingly used to diagnose rare and inherited diseases, particularly when other tests are either inconclusive or simply not available. By examining the entire genome, the test can detect changes that might be missed by other tests, potentially leading to a faster and more accurate diagnosis. Although such tests could help diagnose babies faster and cut healthcare costs, obstacles for implementing the tests include high costs, limited state funding, and the lack of treatments for infants with DM. Experts also discussed ethical concerns—like testing infants for untreatable conditions—but noted that early detection of DM would allow for planning, including engaging in preventive and pre-symptomatic health measures such as regular cardiac monitoring, raise awareness of the serious impact of anesthesia, and would also support research.
Next Steps Toward a Future with Newborn Screening for DM
Participants recommended the following next steps: tracking DM patients over time, piloting health-record studies to identify patients with DM earlier, promoting testing and reimbursement policies, educating medical providers about genome sequencing-based testing and early diagnostics through newborn screening, exploring and advocating for the addition of DM in prenatal screens, and involving the community to build supporting data and resources needed for DM newborn screening in the future.
Thank You to Our Hosts and Organizers
Scientific Summary: Newborn Screening for Myotonic Dystrophy
Background: On February 14, 2025, the RNA Institute and the Myotonic Dystrophy Foundation held a mini symposium to discuss how myotonic dystrophy (DM) could be added to newborn screening. Newborn screening is one of the most successful public health services testing all babies shortly after birth for certain life-threatening diseases. Conditions included in newborn screening are serious and life threatening and without early diagnosis would result in irreparable harm and death. Newborn screening identifies babies at risk before symptoms start, ensures best possible outcomes for all babies, and limits pain and suffering. Methods: The meeting brought together researchers, clinicians, public-health leaders, industry experts, and patient advocates. They reviewed advances in genome sequencing, diagnostic methods, and early pilot newborn screening programs.
Results: Experts agreed that rapid genome testing could speed up diagnosis and help lower healthcare costs. However, several barriers were raised:
- High testing costs
- Limited state funding and a shortage of infrastructure and trained personnel performing genome sequencing based newborn screening
- No existing treatments for infants with DM
Ethical Discussion: Participants debated the ethics of testing newborns for still untreatable conditions. Despite these concerns, they noted that early detection helps parents prepare and supports research progress. Recommendations: The group proposed several action items:
- Follow diagnosed infants over time
- Conduct pilot studies using electronic health records to identify undiagnosed patients
- Advocate for supportive policies and pilot studies ultimately enabling newborn screening for DM
- Educate healthcare providers about DM screening and genetic testing solutions
- Explore adding DM to prenatal screen panels
- Engage families and communities to build support for the adoption of newborn screening for DM
Conclusion: While acknowledging financial, logistical, and ethical challenges, the symposium concluded that early detection of DM offers clear benefits. A coordinated strategy—combining research, policy advocacy, provider education, and community engagement—is essential for future implementation.
Full Technical Summary: Symposium on Genomic Sequencing and Myotonic Dystrophy
Summary of Newborn Screening & Sequencing for Myotonic Dystrophy
Newborn screening (NBS) is a vital and extremely successful public health program aimed at the early identification of conditions that can affect a child’s long-term health or survival. Early detection, diagnosis, and intervention – commonly before the onset of symptoms - can prevent death or disability, alleviate physical, emotional, and economic suffering, and enable children to reach their full potential. With the advancement of genomic sequencing technologies, there is growing interest and opportunity in expanding NBS to include a broader range of genetic disorders. This broadened scope includes conditions, like myotonic dystrophy (DM), for which early treatment may not yet be available but where early diagnosis can inform family planning, clinical monitoring, and guide future therapeutic development. The following summary synthesizes the discussion from a recent Myotonic Dystrophy mini-symposium hosted by the University at Albany’s RNA Institute and co-organized by the Myotonic Dystrophy Foundation and the RNA Institute. The following notes highlight the current state of the field, challenges, and future directions of NBS and genetic testing for DM.
Key Players weighed in on Newborn Screening
The discussions involved a diverse group of stakeholders from academia, industry, public health, and patient advocacy groups. Andy Berglund, PhD (Director, RNA Institute & Chair of Myotonic Dystrophy Foundation Scientific Advisory Committee) and Andy Rohrwasser, PhD, MBA (Chief Scientific Officer, Myotonic Dystrophy Foundation) co-organized and facilitated the meeting with key stakeholders in the field.
In a public session open to the community, key invited experts and opinion leaders summarized critical insights into NBS, myotonic dystrophy (DM), how NBS could be applied to DM, clinical and economic benefits supporting genomics based newborn screening, as well as technology opportunities today that could bridge until genomic NBS solutions would become available.
- Introduction to Myotonic Dystrophy and Therapeutic Landscape; Andy Berglund, PhD; Professor, Director of the RNA Institute, Co-Director, Center of Excellence in RNA Research and Therapeutics, University at Albany, Albany NY
- Introduction to Newborn Screening: How and Why Now? Andy Rohrwasser, PhD, MBA, Chief Scientific Officer, Myotonic Dystrophy Foundation, Oakland, CA
- Introduction to Congenital Myotonic Dystrophy, Nicholas Johnson, MD, MSc, FAAN, Professor, Director of the Center for Inherited Muscle Research, Virginia Commonwealth University, Richmond, VA
- NICU Sequencing: Ultrafast Solution: Scientific Evidence, Economic Sense, Stephen Kingsmore, MD, DSc; President/CEO of Rady Children’s Institute for Genomic Medicine, San Diego, CA
- Pilot Study: Towards Population Wide Sequencing; Wendy Chung, MD, PhD; Chief of Pediatrics, Boston Children’s Hospital, Professor, Harvard Medical School, Boston MA
- Newborn Sequencing in Public Health: How would it work in NY? Michele Caggana, ScD, FACMG; Deputy Director, Division of Genetics; Director, Newborn Screening Program; Department of Health, Wadsworth Center, Albany NY
- Rapid Advance in Analysis and Interpretation; Opportunities in EHR mining - before population wide screening? Mark Yandell, PhD; Professor, Director Eccles Institute Bioinformatics Program; Technical Director, Utah Genome Project; The University of Utah, Salt Lake City, UT
Key Meeting Outcomes and Insights
Following general presentations to the public and interested scientific community members at the University at Albany, the key stakeholders held an in-person discussion with a few virtual attendees. Several important outcomes and insights emerged from the discussion. The Guardian Study was highlighted, as a large-scale genomic screening initiative in New York City, which has screened over 15,000 participants to date and reported a 3.3% positive screen rate with an average turnaround time of approximately 22 days.
Whole genome sequencing (WGS) in neonatal intensive care units (NICUs) was highlighted as the ultimate promise in the ultra-rapid diagnosis reducing time-to-diagnosis and eliminating diagnostic odysseys, but also reducing pain and suffering based on misdiagnoses and associated unnecessary diseases management, while also significantly reducing healthcare costs. The stakeholders also highlighted that repeat expansion disease testing is gaining attention, particularly for conditions such as congenital myotonic dystrophy.
While support for the need for increased testing was strong amongst the group, significant policy and infrastructure challenges were noted. Many states currently lack formal policies for reimbursement for WGS diagnostics, posing a significant fiscal challenge to NBS screening programs. While New York State has pilot programs, there is no statewide policy in place for the selection of disease candidates. Sequencing costs, estimated at around $60 per newborn, along with the need for equipment redundancy and trained personnel, were also raised as significant logistical barriers. The rigorous nature of the Recommended Uniform (newborn Screening) Panel (RUSP) nomination process, which involves a nine-month review process and a 120-day decision window requiring strong evidence of treatment efficacy and public health impact would be significant challenges at this time. This is further complicated by the current administration’s dissolution of the Secretary’s Advisory Committee for Heritable Disorders in Newborns and Children that halted the process and disrupted the mechanism for adding or removing disorders from the recommended panel.
The need for an effective treatment for newborns with DM was discussed as an important point needed to support NBS. Given that clinical trials are currently primarily focused on adults, it was noted that the timeline for approved treatments for newborns was unclear. Foundational to this lack of treatment however is long-term natural history and outcome data for children affected by the early onset form of DM.
Ethical and social considerations were also discussed by the group. Concerns were raised about the implications of screening for conditions without available treatments, including the significant psychological impacts to the affected families and issues associated with insurance and insurability. Clinicians and patient advocates raised the point that early diagnosis can significantly affect family planning and emotional well-being of DM families. There was universal recognition of need for further real-world data and comprehensive, long-term natural history studies, given that many symptomatic individuals remain undiagnosed.
Recommended Next Steps
Several next steps were recommended by the group to advance the field. In terms of research and data collection, more long-term natural history and outcome studies were recognized as essential to understand disease progression and optimal treatment timing. The latter was a key highlight for myotonic dystrophy type 1 (DM1), which is highly heterogeneous in presentation between affected individuals. Integrating real-world data and study data, exploring genotype-phenotype relationships through collaborations with organizations like MDF and academic networks were also raised as crucial next steps. Pilot studies, utilizing EHR mining identifying undiagnosed DM patients for example in the Intermountain Health system, could help assess the feasibility and outcomes of broader implementation and bridge towards the implementation of newborn screening.
From a policy and advocacy perspective, gaining provider buy-in is critical. Here, for example RTI-led focus groups and precedence from other diseases were identified to potentially help to identify provider perspectives and barriers as well as preparing comprehensive evidence packages for RUSP nominations, including cost-benefit analyses and patient impact data. While the likelihood of NBS screening for DM1 was recognized as being years away from reality, the potential of maternal screening initiatives for DM, potentially in partnership with professional organizations like American College of Obstetricians and Gynecologists (ACOG), American College of Medical Genetics and Genomics (ACMG) or Society for Maternal-Fetal Medicine (SMFM) and commercial reference laboratories through integration in existing maternal panels was recognized.
Community engagement was identified as a key focus area raised by the experts and stakeholders alike. The collective group believed that targeted educational materials should be developed to inform families about the opportunities, implications and options related to genetic testing. The involvement of a broad coalition of stakeholders, including families, advocacy groups, and clinicians, was felt to be essential to align priorities and address ethical considerations. The group also believed that current efforts should include ensuring equitable access to testing and support systems for newly diagnosed families.
In summary, while the implementation of NBS for DM faces significant logistics and regulatory hurdles given the lack of an approved treatment approach for newborns affected by the disease, there was recognition of the power, opportunity and necessity of screening and diagnostic sequencing to make an impact on the DM community.