Click here to view the MDFR resarch poster from the 2018 MDF Annual Conference.
Myotonic Dystrophy Family Registry
Report to the Community
December 2017
The Myotonic Dystrophy Foundation launched the Myotonic Dystrophy Family Registry (MDFR) in January 2013 as a tool to help researchers and the myotonic dystrophy (DM) community learn more about the scope and impact of this disease, and to locate and organize the DM patient community for studies and trials. More specifically, the MDFR seeks to:
- Identify, capture and organize potential participants for clinical trials and research studies;
- Accelerate research in DM by collecting information that scientists can use;
- Communicate with the DM community about upcoming research studies and clinical trials;
- Understand how DM can affect the quality of life and circumstances of people and families living with it;
- Help medical professionals improve clinical and self-care for individuals affected by DM.
- Learn how and why certain treatments succeed or fail.
MDFR Overview
The MDFR is an online, patient-reported survey that is organized into three sections. Section 1 focuses on diagnosis, genetic test results, and repeat length. Section 2 contains questions relating to common DM symptoms and treatments. The last section gathers information regarding DM patients’ quality of life and includes questions pertaining to financial impact, employment status and living conditions as they relate to the DM diagnosis.
Each section of the survey incorporates the DM core data elements that were established at the 2009 European Neuromuscular Consortium (ENMC) Workshop. These core data elements allow the pooling of data with other registry studies regarding patient information on demographics, family and general health histories, symptoms and treatments, as well as overall quality of life. For each of the symptoms the survey queries, a follow-up question asks patients if the symptom has a negative effect on their normal daily activities (i.e., symptom burden).
Recent studies through the UK Myotonic Dystrophy Registry, French Myotonic Dystrophy Registry (DM-Scope) and National Registry of Myotonic Dystrophy and Facioscapulohumeral Musclar Dystrophy Patients and Family Members have investigated the prevalence and relative burden of each of these varied symptoms and the resultant impact on quality of life in DM1 and DM2 patients, using patient-reported data, clinical data or a combination of both. As the second-largest DM patient registry in the world, the MDFR provides additional data from more than 1700 patients on the prevalence of symptoms and relative disease burden, the pattern of use of current treatments and medications, and the status of patient quality of life, enriching disease understanding and furthering drug discovery and improvement of patient care.
Figure 1. Growth of MDFR from 2013 – 2017.
(A) Number of patient profiles and
(B) Number of professional profiles
Registry Growth
The growth of patient profiles in the MDFR is shown in Figure 1A. 934 patients registered with the MDFR in the first year. Since then an average of 197 new patients registered annually, leading to 1740 patient profiles at the end of September 2017, or an 86% increase in records since the inception of the MDFR. Of these 1740 patient profiles, 1615 or 93% are complete (i.e., every section was addressed).
Also seen in Figure 1B, the number of professionals using the MDFR has grown by an average of 14 new profiles a year. In September 2017, there were a total of 71 individuals from academia or industry (36 different institutions) that successfully requested access to the de-identified MDFR data, using MDFR data for research studies (e.g., the pregnancy study published by the University of Utah), to help recruit patients for 7 clinical trials and research studies and to communicate with patients. The MDFR has been cited in two publications, and one draft publication has been developed by MDF staff that outlines the DM1 and DM2 population in the MDFR. A large number of requests to access the MDFR data are also routinely turned down, because the proposed uses are not compelling or credible, or because the requesting professional or organization intends to sell the data to industry or other third parties (e.g., CROs).
Patient Demographics
Of the 1740 patients in the MDFR, 120 patients have juvenile onset DM, 237 patients have congenital DM, 278 have DM2 and 783 have DM1 according to their doctors. Additionally, 1212 of these patients have had their diagnosis confirmed via genetic testing. 120 patients responded that they do not know what their diagnosis is and an additional 202 patients left this question blank or did not complete this portion of the survey. There are 882 females (50.7%) and 806 males (46.3%), with an additional 52 that did not provide this information. Approximately 90% of the patients within the MDFR are white.
MDFR Study and Publication
As mentioned above, numerous studies have been published using data from other global DM registries. All these studies have focused on DM1 and DM2. MDFR data can be used to expand upon the knowledge gained from other DM registries, particularly since the number of DM1 and DM2 patients evaluated in this study is higher than other studies previously reported. The MDFR has prepared its first draft publication for a peer-reviewed journal using patient-reported data from the MDFR to evaluate the DM1 and DM2 populations. The large number of records and data in the MDFR and the consistency with other registry data and published findings makes published information from the MDFR a useful and credible addition to the literature.
Draft Publication - Participant Demographics and Genotype
The MDFR registry review included registry participants who: 1) were age of 18 years or older, 2) had a diagnosis of adult-onset DM1 or DM2 through clinical examination (diagnostic tests, such as electromyography, heart and lung monitoring tests, etc.) or genetic testing and 3) had a complete profile in the system (answered 100% of the questions). Patients that reported a diagnosis of juvenile-onset or congenital DM were not included in this study; MDFR staff plan to analyze these data separately. All US and international patient records were evaluated for eligibility. Only data from patients with completed surveys were included in the review, hence approximately 7% of registrants were excluded from the study. At the time analyses were conducted, there were 630 eligible DM1 patients and 224 eligible DM2 patients. 566 of the 1420 registry participants at that time were excluded based on the criteria listed above. Of the 854 patients analyzed, 86% resided in the United States. The remaining 14% were from other areas of North America (Canada and Mexico), Europe, Asia and Australia.
Of the 630 DM1 patients, there was an equal distribution of males and females, with an additional 0.6% of the DM1 population that did not disclose their gender. The mean age for the DM1 group was 48 years of age (range 18-89), with 31 years as the average reported age of symptom onset. 84.8% of the 630 DM1 patients reported that their diagnosis was confirmed via genetic testing. The remaining 15.2% were diagnosed using clinical criteria as defined in the methods section. Additionally, BMI was calculated from the patient’s reported weight (pounds) and height (inches).
For the DM2 group, 89.8% of the diagnoses were confirmed via genetic testing. 53.1% of the 224 eligible DM2 patients were female. The average age of the DM2 group was 54 years (range 20-89+), with onset of symptoms occurring at an average of 36 years.
Draft Publication – Prevalence and Symptom Burden
Average negative effect on patients’ normal daily activities (a metric with range from 0 to 2) was determined for each symptom that was assessed in the survey. Responses were categorized for the total DM1 and DM2 populations, and then subcategorized according to gender and age (18-30y, 31-40y, 41-50y, 51-60y, and 61+y). Descriptive statistics for all categories and subcategories were obtained. In order to determine the symptomatic themes that are significantly more prevalent in males versus females, or across the different age ranges, a one-sample proportions test (a statistical tool designed to determine whether the proportion of a population with a given trait is significantly different from a hypothesized value) was used to compare prevalence of each theme across the different subgroups. An Analysis of Variance (ANOVA; a statistical tool designed to analyze differences among group means—for example, whether there is a difference in the averages for a cardiac function measure are similar or different in males versus females) was used to compare the average negative effects (i.e. most burdensome) across the different groups to evaluate significant gender or age differences within the DM1 and DM2 patient populations. Post hoc analysis of significant effects was conducted. MDF staff conducted comparison analyses against control conditions using Bonferroni test, evaluated at α=0.05.
The most prevalent symptom for both DM1 and DM2 included limitations in doing vigorous activities, such as exercise, (85.4% and 91%, respectively, Table 1). For DM1, symptoms with the next highest prevalence included fatigue (79.4%), myotonia (78.9%) and impaired sleep or daytime sleepiness (76.2%) (Table 1). This differed from DM2 patient reports, which identified the next most prevalent symptoms as pain (74.7%), fatigue (73.8%) and limitations with mobility or walking (72.4%). Reports of difficulty breathing, diabetes, heart conditions and problems choking or swallowing were significantly higher in DM1 and DM2 males than females. There were no differences in the BMI between males and females with DM1.
Table 1. Prevalence and relative impact of symptoms in DM1 and DM2.
*p<0.05, **p<0.01, ***p<0.005 compared to females; +p<0.05 vs 18-31y; ^p<0.05 vs 31-40 y; #p<0.05 vs 41-50 y; $p<0.05 vs 51-60y.
Additionally, data from DM2 patients demonstrated that the following symptoms were more prevalent in males than females:
- Limitations with mobility or walking
- Fatigue
- Psychiatric disorders
- Emotional or behavioral problems
Prevalence data were also stratified into five age groups (18-30y, 31-40y, 41-50y, 51-60y, 61+y) and the most common symptoms for each DM1 and DM2 group were reported. For both DM1 and DM2, prevalence of limitations with mobility or walking significantly increased with age (Figure 2), as did heart complications and cataracts (15.9% to 53.7%; p<0.001). In the DM1 population, problems choking or swallowing also increased with age (Figure 2). The prevalence of psychiatric disorders and emotional or behavioral problems, reported by both DM1 and DM2 patients, decreased between the ages of 35 and 40. This decrease in prevalence may be associated with structural and functional changes that occur in the CNS of both DM1 and DM2 patients. A 9-year longitudinal study by Gallias et al. demonstrated a cognitive decline in adults with DM1 leading to a decline in executive functions, language and visual memory. It may be possible that the decline in cognitive function leads to a decrease in psychiatric and emotional or behavioral problems as the CNS, however more studies are warranted to strengthen this correlation.
The most burdensome symptom, or that with the most negative effect, in the total DM1 and DM2 population was limited ability to carry out vigorous activities (1.38±0.71 and 1.50±0.63, respectively; Table 1). Interestingly, this particular symptom was cited by both DM1 and DM2 patients as the most prevalent symptom (see above).
In DM1 patients, fatigue (1.15±0.70), impaired sleep or daytime sleepiness (1.12±0.71), and myotonia (1.06 ±0.66) (Table 2) were the most burdensome symptoms after limitations in vigorous activity that had a negative effect on the patients’ daily lives, whereas DM2 patients reported that limitations with mobility or walking (1.13±0.79), pain (1.11±0.76), and fatigue (1.10±0.73) were the next most burdensome symptoms (Table 1). Males with DM1 and DM2 self-reported as significantly more burdened than females with DM1 with the following symptoms:
- Limitations with mobility or walking
- Myotonia
- Difficulty breathing.
Comparing the DM1 overall burden across the different age ranges, significant differences were seen only with regard to limitations with mobility or walking, with the burden increasing with age (0.43±0.55 to 1.24±0.80; p<0.001). For DM2 patients, the negative impact associated with limitations with both mobility or walking and impaired sleep or daytime sleepiness also significantly increased with age.
Figure 2. Changes in the prevalence of DM1 (A) and DM2 (B) symptoms with age. 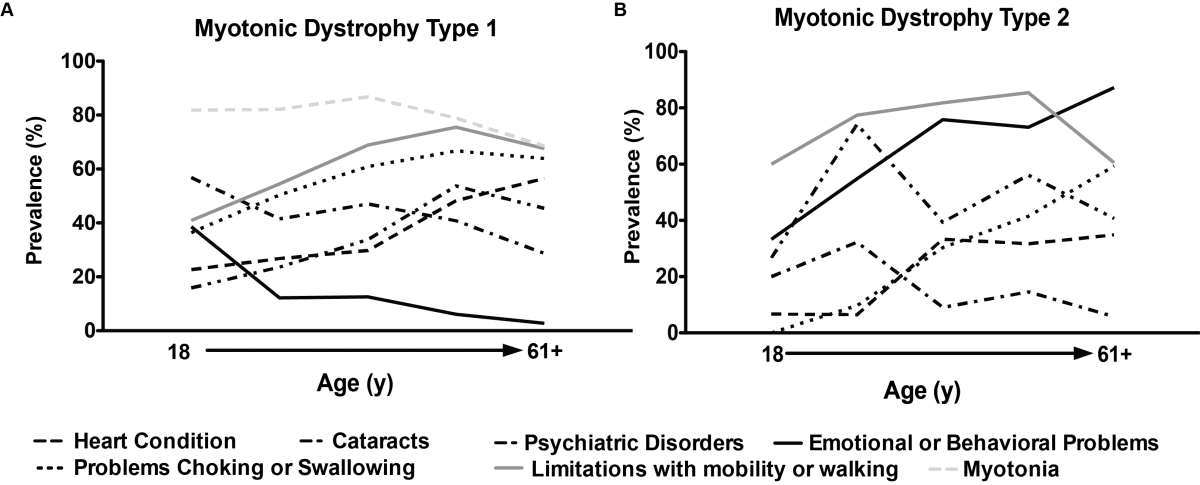
Figure 3. Correlation of the type of health insurance with in the DM populations. 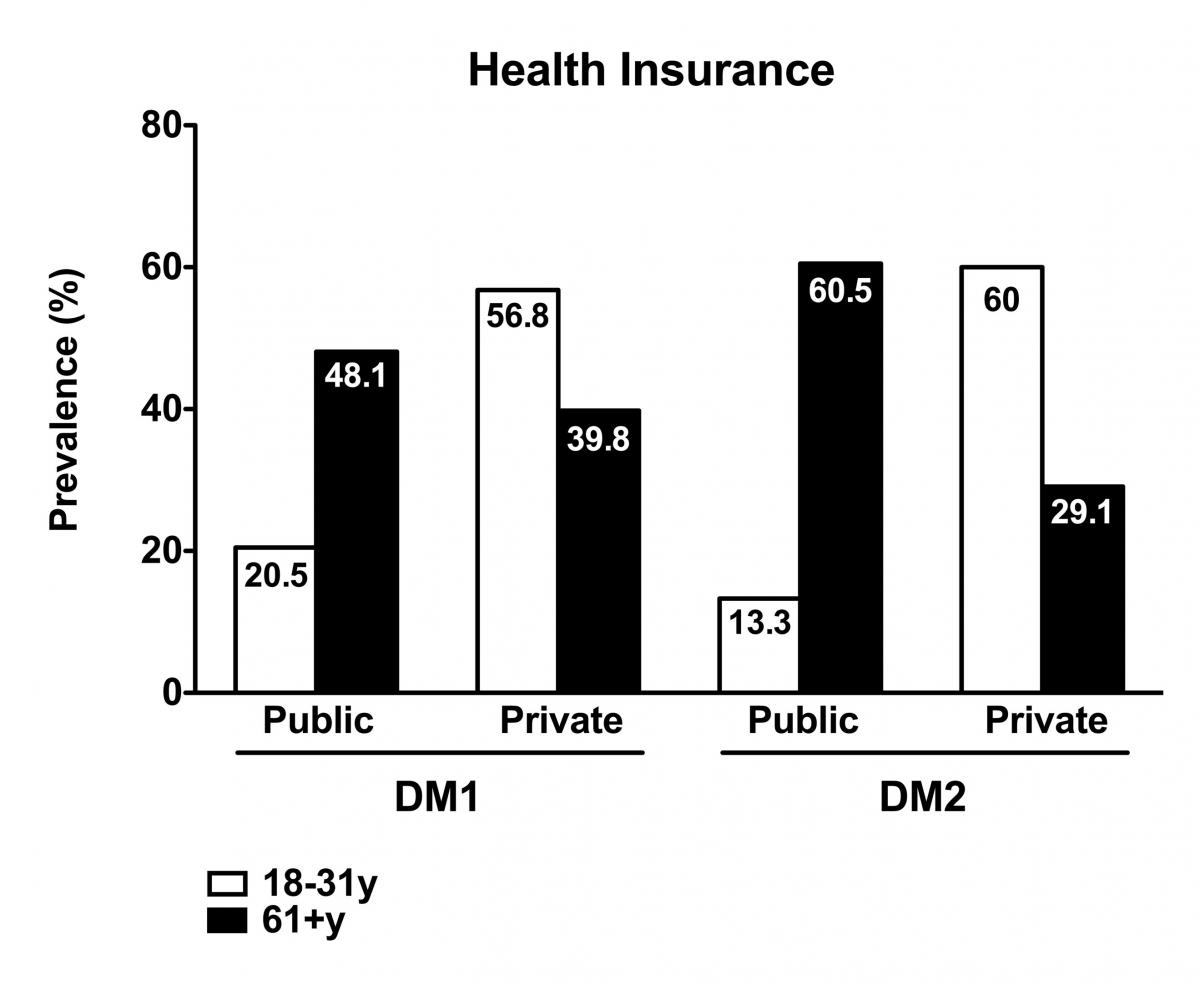
Draft Publication – Health Insurance Usage
As seen in Table 2, 64.3% of DM1 patients reported having some financial impact due to their DM1 diagnosis. Interestingly, males reported more of a financial burden than females with DM1 (1.07±0.81 versus 0.90±0.76; p<0.001). For DM2 patients, 55.7% of patients reported a financial impact, however, no differences were seen when comparing genders.
In DM1 patients, 28.6% were disabled or unable to work, while 26.2% had full-time employment status. The remaining DM1 patients were evenly dispersed between part-time employment, unemployed and retired. When comparing across the two genders, males were more likely to hold full-time employment status (29.8% vs. 22.3%; p<0.05), were more likely to be disabled/unable to work (35.1% vs. 22.3%; p<0.001), or were more likely to be retired (14.4% vs. 8.6%; p<0.05). On the other hand, a higher percentage of females held part-time employment (16.7% vs. 9.8%; p<0.05) or were unemployed (19.5% vs. 6.7%; p<0.001). In the survey, we asked their employment status and gave them 5 options: Full-time, Part-time, Retired, disabled/unable to work, and Unemployed. Males were high in Full Time, Disability and Retired, while females were higher in Part-time and unemployment. No correlations can be made regarding whether unemployment was related to disability since subjects could only select one answer.
Looking further into the employment status data, 57.6% of the total DM1 population reported that their diagnosis impacted their employment in the following ways:
- 31.3% of the total DM1 population claimed disability
- 17.0% reported that they had lost their jobs
- 9.5% needed job accommodations
- 9.4% took early retirement.
Further analysis showed that males lost their job or claimed disability more frequently than females (21.4% vs 12.8%; p<0.001 and 40.4% vs. 22.8%, p<0.001, respectively). Of the 42.4% of the DM1 population whose employment status was not impacted by their DM1 diagnosis, 48.7% were female (p<0.001).
The employment status for DM2 patients (Table 2) showed similar trends to those in the DM1 population, with no significant differences found between DM types. Like DM1, 58.4% of the total DM2 patients reported that their employment status had been affected. 27.1% of these patients claimed disability, 14.9% had taken early retirement, 12.2% had lost their jobs, and 11.3% needed job accommodations. Again, males had a higher tendency to lose their jobs (18.1% vs. 6.7%; p<0.05) or claim disability (33.3% vs. 11.3%). However, 31.4% of those not impacted were male (p<0.01 compared to 26.7% of females), which differed from results seen in the DM1 population. For both DM1 and DM2, full-time employment decreased with age, and there was an increase in early retirement.
For both DM1 and DM2 patients, a majority reported that they were capable of taking care of themselves (51.1% and 57.9%, respectively), while the remainder depended on a family member, spouse, or professional. For both DM1 and DM2, a higher percentage of those who reported as self-dependent were females, whereas the males tended to rely on others more frequently. Self-dependence declined with age.
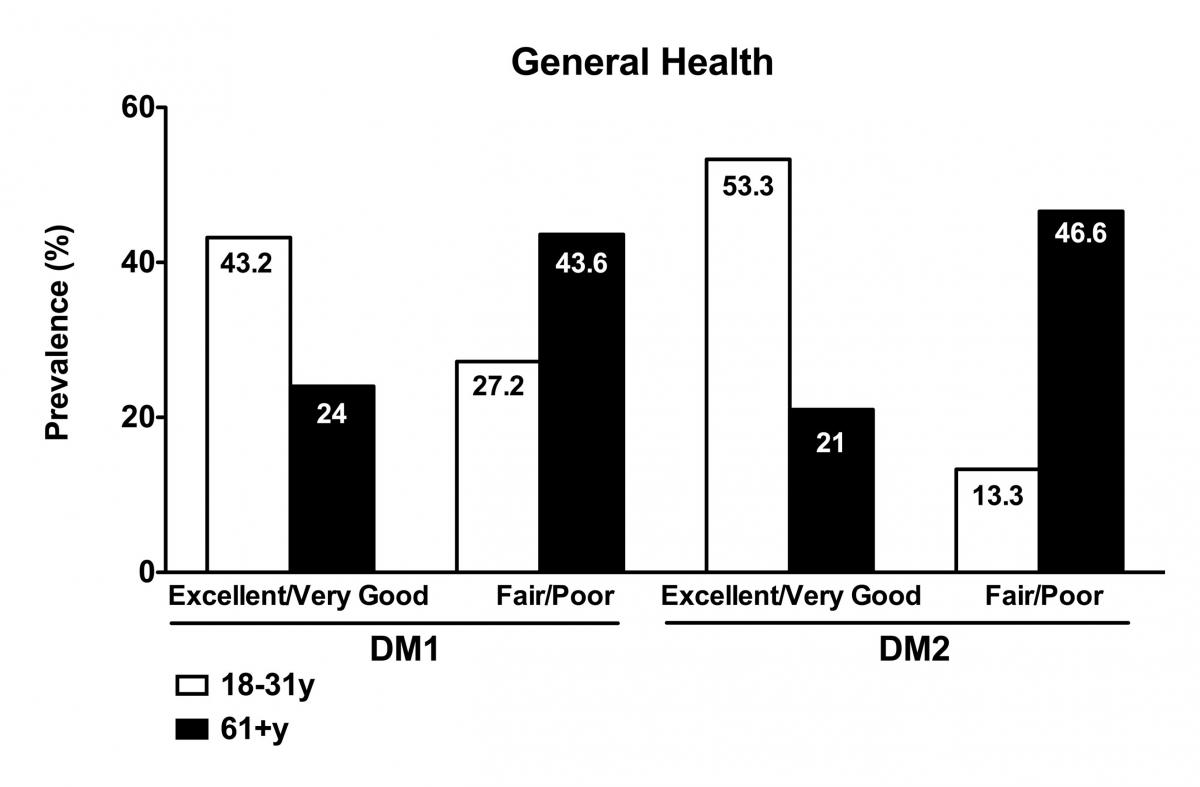
A minority of DM1 and DM2 patients reported their overall health to be good (33.7% and 33.9% respectively). In DM1 patients, 21.7% of the patients reported having excellent or very good general health, while 42.1% reported fair or poor general health. 24.1% of females compared to 17.2% of males reported they were in excellent or very good health (p<0.001; Table 2), while 49.8% of males with DM1 compared to 34.3% of females with DM1 were in fair or poor health (p<0.001). The trends were the same for DM2 patients (Table 2). 23.5% of the total DM2 population reported having excellent or very good health, while 38.4% were in fair or poor health. No gender differences were found in the DM2 population. DM1 and DM2 patients showed a decline in health with age, from excellent/very good to fair/poor (Figure 4).
Table 2. Quality of Life in DM1 and DM2.
*p<0.05, **p<0.01, ***p<0.005 compared to females; +p<0.05 vs 18-31y; ^p<0.05 vs 31-40 y; #p<0.05 vs 41-50 y; $p<0.05 vs 51-60y.
Draft Publication – DM1 & DM2 Treatment Approaches
Table 3 illustrates the treatments DM1 and DM2 patients report using to manage their symptoms. There is a high prevalence of the use of orthopedic devices in DM1 and DM2 patients (75.2% and 88.2%, respectively), and usage increases among those reporting limitations with mobility or walking (prevalence exceeding 100 signifies that patients used more than one orthopedic device). Prevalence of surgery to treat cataracts was also high in both DM1 and DM2 patients (36.3% and 37.6%, respectively). A small percentage of DM1 and DM2 patients who report myotonia take medication (Mexiletine, Phenytoin, Carbamazepine, etc.) for symptom management. The use of non-narcotics to manage pain, and heart medications, such as β-blockers, was also common in both DM1 and DM2 patients.
Table 3. Top four prevalent and most burdensome symptoms in the DM1 and DM2 populations in the MDFR compared to the published literature.6,7,19,20
Draft Publication - Discussion
The symptoms, severity of illness and age of onset in both DM1 and DM2 patients have been shown to vary greatly between individuals, which has been partially responsible for the difficulties experienced in the development of a standardized treatment and care program for these populations. The primary goal of this study was to use data from a patient-reported registry (MDFR) in order to assess the prevalence of symptoms and relative burden in DM1 and DM2 patients, as well as the pattern of utilization of current treatments and medications and patient quality of life. Heterogeneity in the presentation and progression of DM, a well-recognized feature of the disease, complicates both patient management and clinical trial design and conduct.
Data from the MDFR can help to better characterize the phenotype and heterogeneity of DM1 and DM2 to focus patient care (including the type, timing and frequency of recommended medical examinations) and development of novel therapeutics for symptoms that are clinically meaningful to DM patients. Moreover, detailed knowledge of the symptomatology and impact of DM can support efforts to advocate on behalf of people living with DM to insurers and government agencies.
Overall, the data in the MDFR registry review provide a better understanding of DM1 and DM2 and the impact these diseases have on the daily lives of those afflicted. For both DM1 and DM2 patients, limited ability to carry out vigorous exercise was the most prevalent and burdensome symptom. For DM1 patients, fatigue was reported to be the second common and taxing symptom (79.4% with a burden score of 1.15), thus suggesting a relationship between propensity to fatigue and limitations in vigorous activities. However, for DM2 patients, such limitations in physical activity may be secondary to pain, which is the symptom most frequently reported after limitations in doing vigorous activities, as well as one of the top three most burdensome symptoms.
Taken together, these findings provide insight into possible treatment paradigms that can be developed to address the connections between symptoms, rather than treatment of each individual symptom. For example, fatigue, daytime sleepiness, and cognitive issues experience in DM1 are interrelated and might be addressable by repurposing drugs currently in use to treat other sleep disorders. Going forward, new candidate therapies that address the primary disease mechanisms in DM may well address a broad spectrum of symptomatology, but only if they account for delivery to the multiple organ systems involved.
Draft Publication – Study Limitations
Limitations in this study include the fact that data was taken at one time point and that individual patient profiles were not analyzed (repeat size, comorbid conditions, severity of illness, etc.), thus age ranges chosen do not necessarily represent disease progression but rather a snapshot of the disease within those respective ages. Additionally, we cannot conclude that the patients participating in MDFR are a true representation of the broader DM1 and DM2 populations; they are typically those with a less severe disease form who are thus more engaged and willing to participate in an online registry. While more work is needed to gather longitudinal and more complete data, and to develop new therapy and treatment strategies to enhance the quality of life for those affected by DM, the MDFR registry review shows that patient-reported registries can be instrumental in providing data to support development of proper care guidelines and better design and conduct of interventional clinical trials.
Comparison of MDFR to Other DM Registries
The French DM1-Scope, UK Myotonic DM Patient Registry and University of Rochester registry also reported clinical observations on the timing of symptom occurrence, providing a comparator for the MDFR DM1 patient-reported data. Additionally, to this date, much of the patient-reported research regarding the prevalence and consequences of symptoms pertaining to DM1 and DM2 has stemmed from the analysis of the National Registry of Myotonic Dystrophy and Facioscapulohumeral Musclar Dystrophy Patients and Family Members that is maintained at the University of Rochester (URC). Comparison of participants between the URC registry and MDFR utilizing global unique identifiers (GUIDs) revealed less than 1% overlap between participants in these two registries. The data analyzed from the MDFR is closely aligned with the previously published datasets from the URC registry (Table 4), despite potential demographic differences. Taken together, the results from our study and these prior studies draw similar conclusions regarding the prevalence and burden of symptoms in DM and the collective data are useful to bridge the gaps in the management and treatment of DM1 and DM2.
Future Directions
MDF engaged PicnicHealth (PH), a San Francisco-based, consumer-facing digital medical record management company, in December 2016 to conduct a pilot study to collect comprehensive medical records from MDFR participants. MDF’s objective was to ascertain whether longitudinal clinical data from patient records, collected by various clinicians in numerous locations, could supplement efforts to develop better understanding of disease onset, progression and severity. The pilot study was funded by Biogen. At MDF’s invitation, thirty-nine patients from the MDFR were consented and enrolled in the pilot study . Of these 39 patients, all were self-identified in the MDFR as having DM1. However, 2 of the 39 had medical records that documented DM2. These two patients were excluded from the data analysis. The average age of the remaining 37 patients was 51 years old (range 20-72). Of the 37 patients, there were 18 males and 19 females.
Medical records with an average time span of 6 years per patient (range 1-18) were collected, with multiple visits per year. Visit types from which medical records were pulled from include:
- Procedure (ECG testing, Echocardiogram, Biopsies, Auditory exams, Holter tests, etc.)
- Correspondence visits (mail, telephone or email consult)
- Genetic Screen
- Inpatient (Hospitalizations)
- Lab Tests
- Outpatient (Office visits – Cardiology, Gastroenterology, Neurology, Family Medicine, Optometry, Psychiatry, Genetics, etc.),
- Pathology Study (Culture, Cytology, Pathology)
- Radiology (MRI, ultrasound, X-ray, etc.)
Next steps
MDFR staff plan to analyze the data pulled from the custom abstraction to create a longitudinal picture of the medical history of the DM1 population. Data analysis will be based upon standard abstraction. MDF staff plan to use medical record data endpoints, obtained via the standard abstraction method, to corroborate existing patient entered information in the MDFR. The custom data abstraction should also be informative in addressing specific organ system involvement (e.g., we will be able to evaluate an observed drop in systolic blood pressure with age as a function of a change in the electrical or structural properties of the heart via electrocardiogram and echocardiograms data).
MDF hopes the novel approach used and these data can be published in a peer-reviewed journal. MDF is also evaluating strategies for increasing the number of publications from the MDFR, both from staff and increasing visibility of the MDFR resource for analyses by and publications from other academic researchers.